[新しいコレクション] heterogeneous mixture of elements 719163-A heterogeneous mixture of elements and compounds
In chemistry, a heterogeneous mixture consists of either or both of a) multiple states of matter or b) hydrophilic and hydrophobic substances in one mixture; Examples of the heterogeneous mixture – Most of the mixtures occurring in nature are heterogeneous For example, the soil is a mixture of hundreds of elements and compounds Its composition changes from place to place Some other examples of the heterogeneous mixture are – rocks, a mixture of kerosene and water, rice and pulses, etcGroup 1A (or IA) of the periodic table are the alkali metals hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr)

Chapter Two Properties Of Matter Matter Pure Substance Elementcompoundmixture Homogeneous Mixture Solution Heterogeneous Mixture Colloidsuspension Classification Ppt Download
A heterogeneous mixture of elements and compounds
A heterogeneous mixture of elements and compounds-Chemistry Lesson 15Types of mattermixtures vs pure substanceshomogeneous vs heterogeneouselements vs compoundsalloyscompositionpropertiesseparation technAn alloy is a mixture of elements that has the characteristic of a metal At least one of the elements mixed is a metal One example of an alloy is steel which is made from a mixture of iron and carbon Suspensions (heterogeneous) A suspension is a mixture between a liquid and particles of a solid In this case the particles do not dissolve
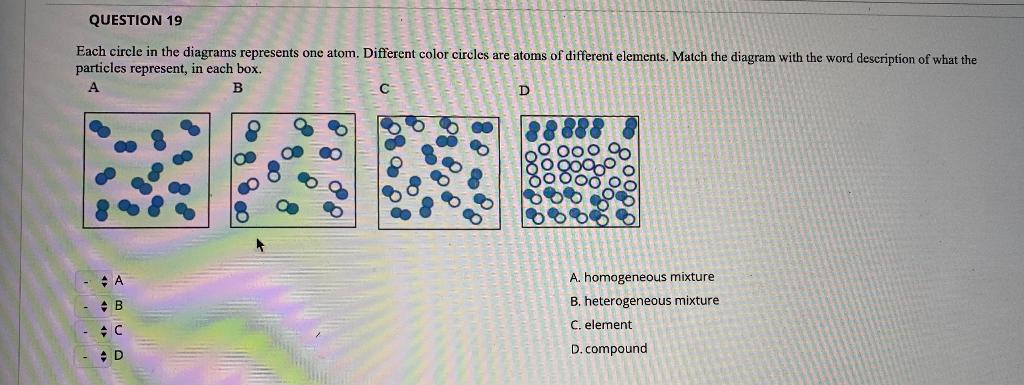



Question 19 Each Circle In The Diagrams Represents Chegg Com
When two or more elements or compounds mix together, not necessarily in a definite ratio and do not interact chemically, then the resulting substance is know All heterogeneous mixtures are colloid and suspension Mixtures in which substances remain distinct and one substance disperses into another substance in the form of small bubbles or in any form are called heterogeneous mixtures For exampleIce cubes in cola form a heterogeneous mixture Fruit and nut cookies are a heterogeneous mixtureClassify the following materials as substances or mixturestable salt, wood, mercury, air, water, seawater, sodiumchloride, and mayonnaise If they are mixtures, subclassifythem as homogeneous or heterogeneous;
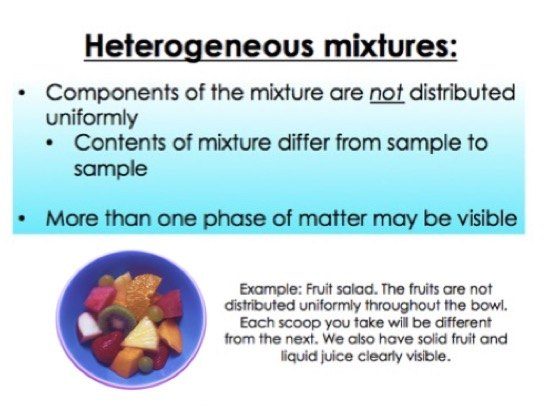
A heterogeneous mixture is often composed of different solids, or of matter existing in two or more different phases Phases are states of matter; Types of heterogeneous mixtures Solid Soil, fruit baskets, rice with lentils, and minerals in many colorful crystals are examples of solid Suspensions The expression 'stars suspended in the sky', helps to understand what suspensions are This type of Colloids What if, instead of sand, much A mixture is a material containing two or more elements or compounds that are in close contact and are mixed in any proportion For example, air, sea water, crude oil, etc The constituents of a mixture can be separated by physical means like filtration, evaporation, sublimation and magnetic separation
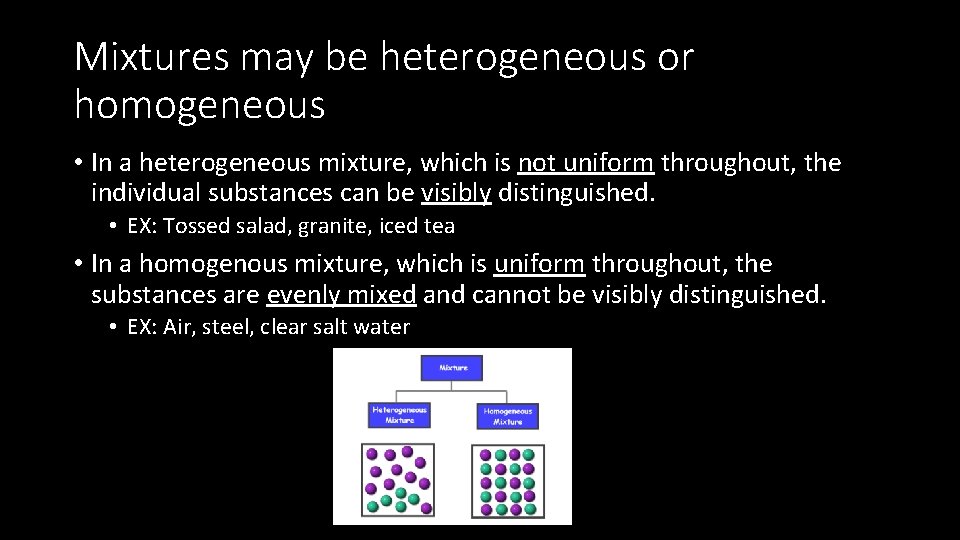
An element is a substance that is made entirely from one type of atom For example, the element hydrogen is made from atoms containing a single proton and a single electron Heterogeneous mixtureThe mixture in which the components mixed are not uniformly distributed in the mixture is called heterogeneous mixture The mixing components can A heterogeneous mixture has uneven distribution of its components Breakfast cereals are good examples of heterogeneous mixtures (think of the raisins and flakes in a raisin bran cereal) Is stainless steel a heterogeneous mixture? A heterogeneous mixture is a mixture with a nonuniform composition The composition varies from one region to another with at least two phases that remain separate from each other, with clearly identifiable properties If you examine a sample of a heterogeneous mixture, you can see the separate components
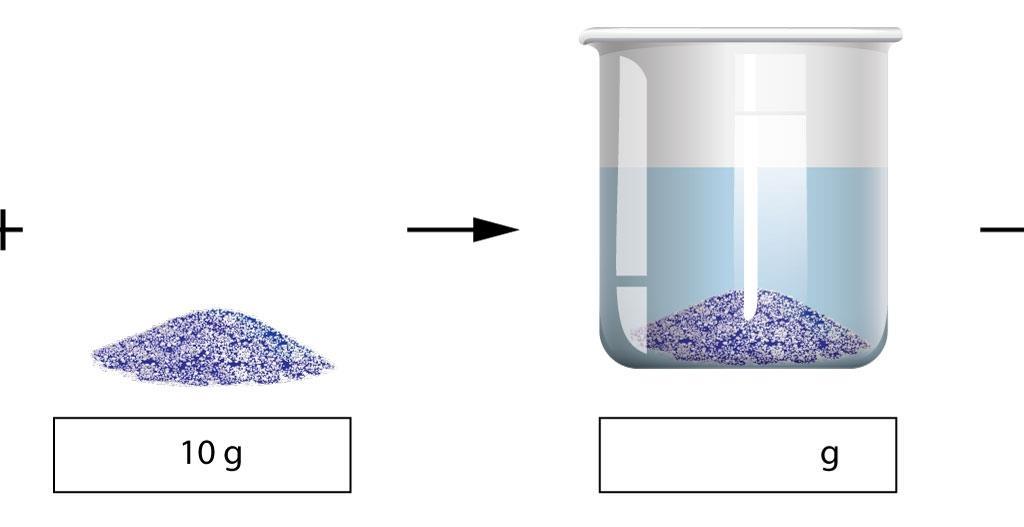



Mixtures And Solutions Cpd Rsc Education
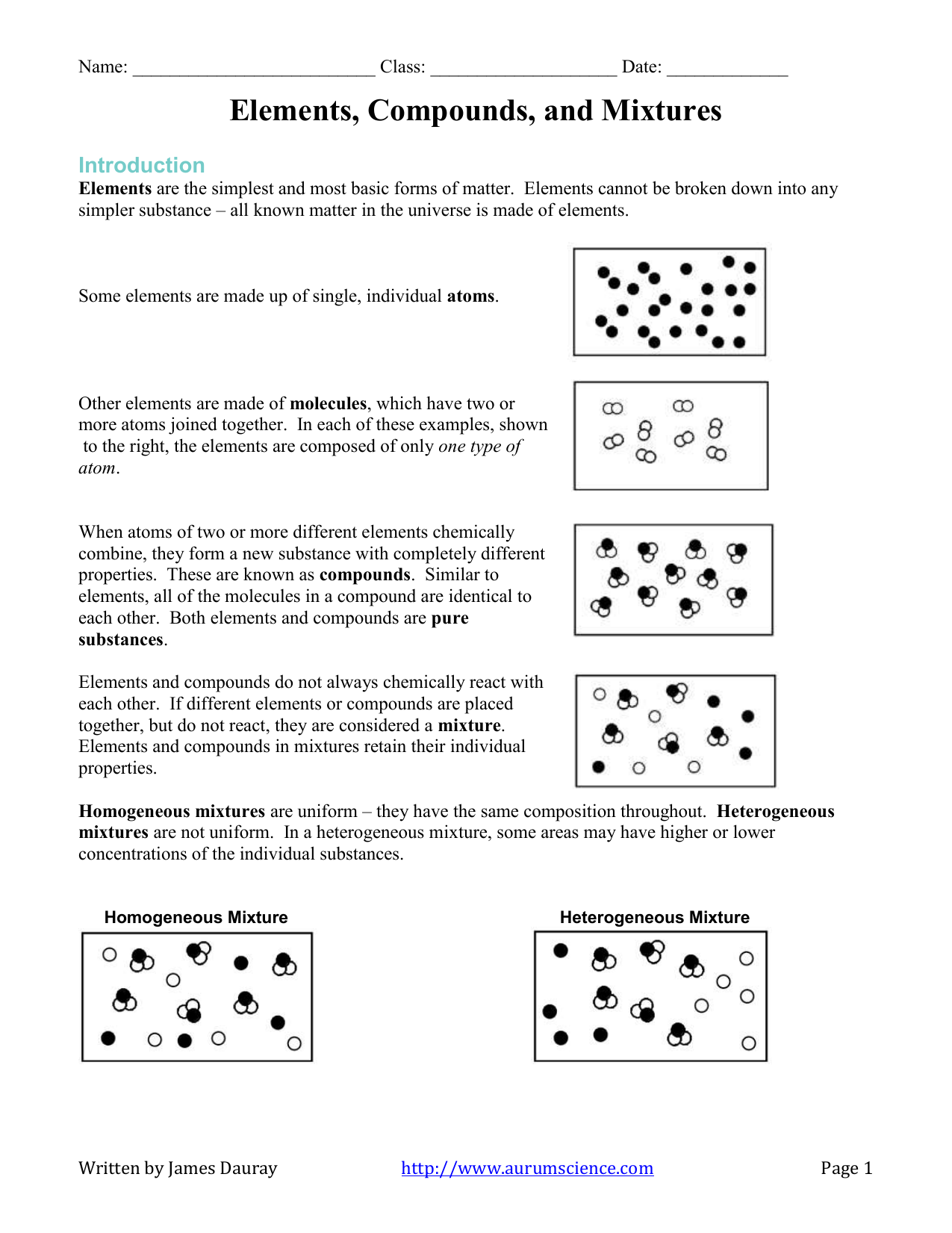



Elements Compounds And Mixtures Worksheet
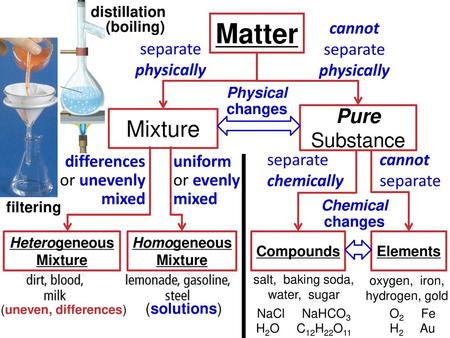
Explain the difference between a homogeneous mixture and a heterogeneous mixture Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture Matter is anything that has mass and volume Nearly everything within the known universe, from water, to a fish, to the planets, is composed of matterMixtures can vary a lot and can be homogeneous or heterogeneous Homogeneous mixture Mixtures involve mixing substances, so let us first be clear about what a homogenous substance is When a sample of matter has the same composition throughout, we call that substance a homogeneous substanceElements, Compounds, and Mixtures An element is a pure substance made up of atoms with the same atomic number A compound is a pure substance made up of two or more elements
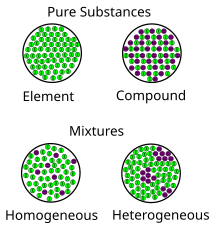



Mixture Wikipedia




Difference Between Mixtures And Compounds In Tabular Form
Heterogeneous definition, different in kind; The air in the atmosphere consists of nitrogen, oxygen, which is the lifesustaining substance for animals and humans, carbon dioxide, water vapor, and small amounts of other elements (argon, neon, etc) Higher in the atmosphere air also contains ozone, helium, and hydrogen People can only clearly notice the presence of air when the wind blows Soil is a heterogeneous mixture because it is comprised of various minerals and elements arrayed in a nonuniform fashion Soil frequently has clumps with higher concentrations of a mineral than the area immediately surrounding that clump of soil




Zia Learning Elements Compounds Mixtures




Nia S Stuff Chemistry Homework 4
Is alcohol heterogeneous mixture?Element and Compound are examples of pure substances Mixtures are made up of two or more substances which are the constituents of mixture The constituents of mixture can be in any ratio Mixtures can be divided into 1 Homogeneous mixtures 2 Heterogeneous mixtures Homogeneous mixtures have uniform composition For instance, sugar inConcrete is a heterogeneous mixture It is composed of cement, water, and aggregate that can be crushed stone, sand, and/or gravel Concrete is made up of different compounds such as solids – aggregate – and liquid – water This mixture of various materials makes it heterogeneous One of the most important things to remember about
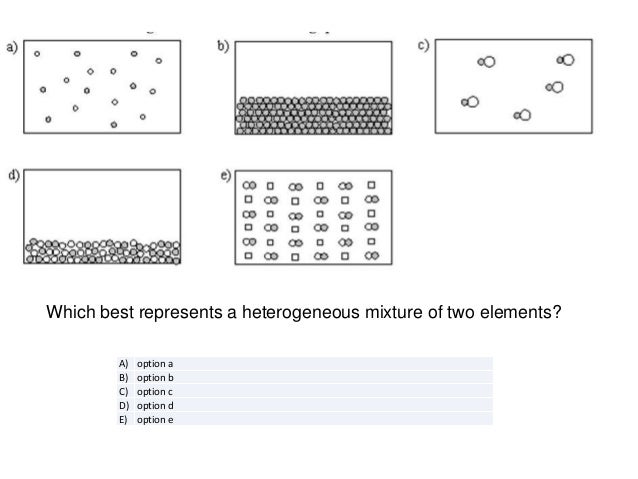



Chapter 1 Review




10 Examples Of Mixtures
Pure substances can be elements or compounds Mixtures vs Compounds Mixtures are constituted by more than one kind of pure form of matter, known as a substance A substance cannot be separated into other kinds of matter by any physical process Differences between homogeneous and heterogeneous mixtures Definition of Heterogeneous Mixtures A mixture is a combination of two or more pure substances in which the original substances retain their chemical properties In some mixtures, the initial A heterogeneous mixture is a mixture of two or more chemical substances (elements or compounds), where the different components can be visually distinguished and easily separated by physical means What are 2 ways in which mixtures differ from compounds?




Test bank general organic and biological chemistry 7th edition By Ronaldperez Issuu




Elements Compounds Mixtures Objectives 1 Explain The Difference Between An Element And A Compound 2 Compare Heterogeneous And Homogeneous Mixtures Ppt Download
Mixtures Matter Substances Elements Compounds Mixtures Heterogeneous Mixtures Homogeneous Mixtures Substances separated by physical methods Compounds Substances separated by chemical methods Mixtures • Combo of 2 or more pure substances • Physically combined NOT chemically combined • Each substance retains its own identity and properties Define element, mixture and compound Explain the differences between pure substances and mixtures Explain the characteristics of the mixtures Give some examples of elements, mixtures and compounds Define the homogenious and heterogeneous mixtures and give some examples Explain in general how mixtures can be separated A mixture is heterogeneous or homogeneous What is another name for the group A elements?



Homogeneous Vs Heterogeneous Matter Worksheet Worksheet List




What Are Examples Of Pure Substances
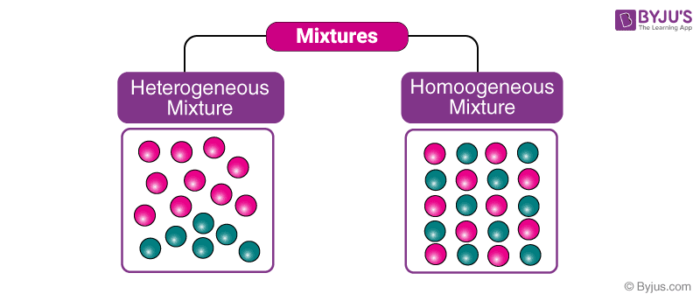
8)A solution is a homogeneous mixture of two or more components with a smaller size than 1 nm Homogeneous mixtures have only one phase Homogeneous mixtures – Seawater, air and soda water Heterogeneous mixtures – Coal and Soil 9) (b) milk and (d) starch solution show Tyndall effect because they are colloidal solution Answer d Saltwater acts as if it were a single substance even though it contains two substances—salt and water Saltwater is a Soil is composed of small pieces of a variety of materials, so it is a heterogeneous mixture Water is a substance More specifically, because water is composed ofHomogeneous Mixtures in which the two or more substances that form the mixture are evenly distributed throughout the mixture, eg vinegar is a homogeneous mixture of ethanoic acid and water Heterogeneous Mixtures in which the two or more substances that form the mixture are not evenly distributed throughout the mixture, eg oil and water
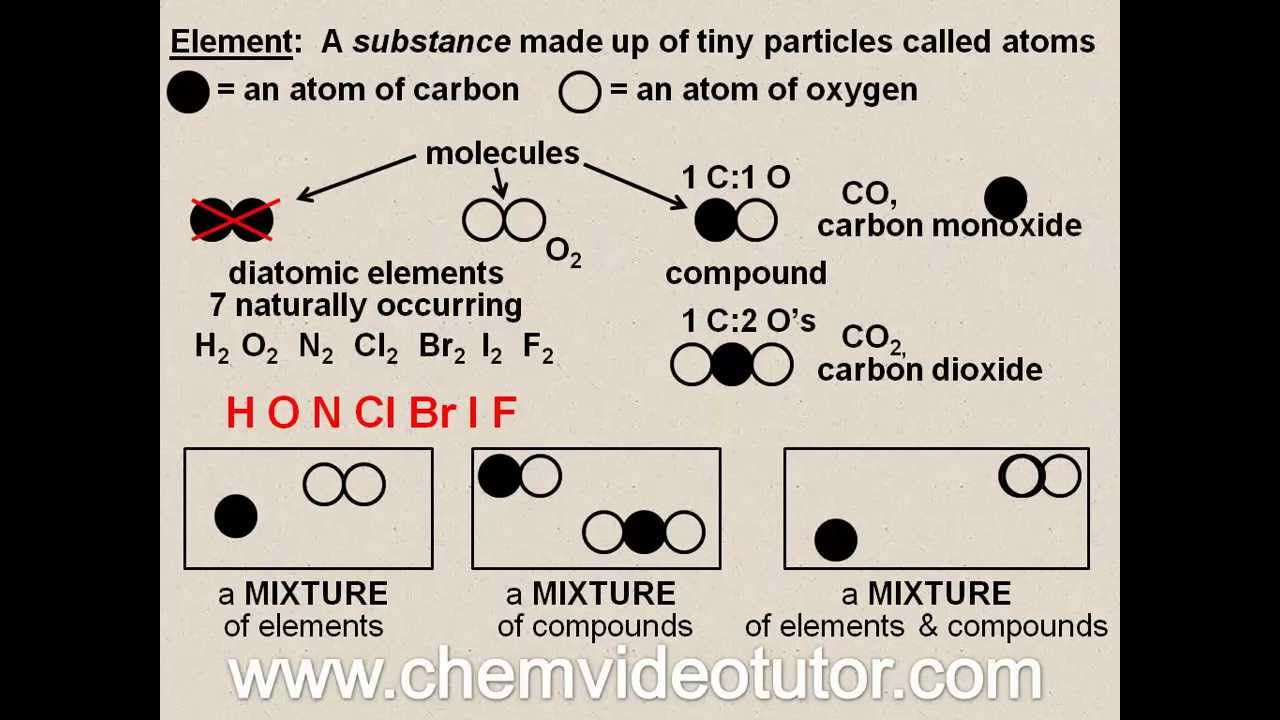



Elements Compounds Mixtures Oh My Youtube
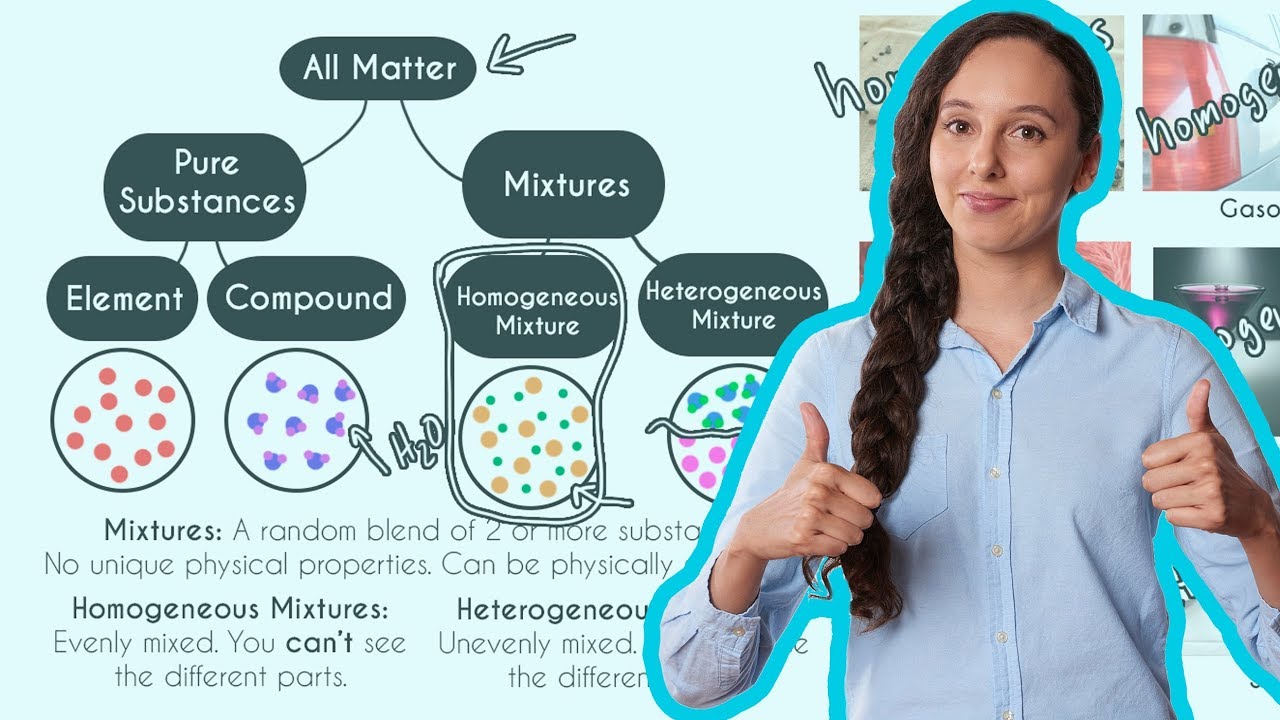



Homogeneous And Heterogeneous Mixtures Youtube
Start studying Element, Compound, or Homogeneous or Heterogeneous mixture Learn vocabulary, terms, and more with flashcards, games, and other study toolsYes, it is a type of homogeneous mixture It is because it has uniform composition (wood pulp) throughout the mixtureAnswer A 70% alcohol is a homogeneous mixture Is wood a compound element or mixture?
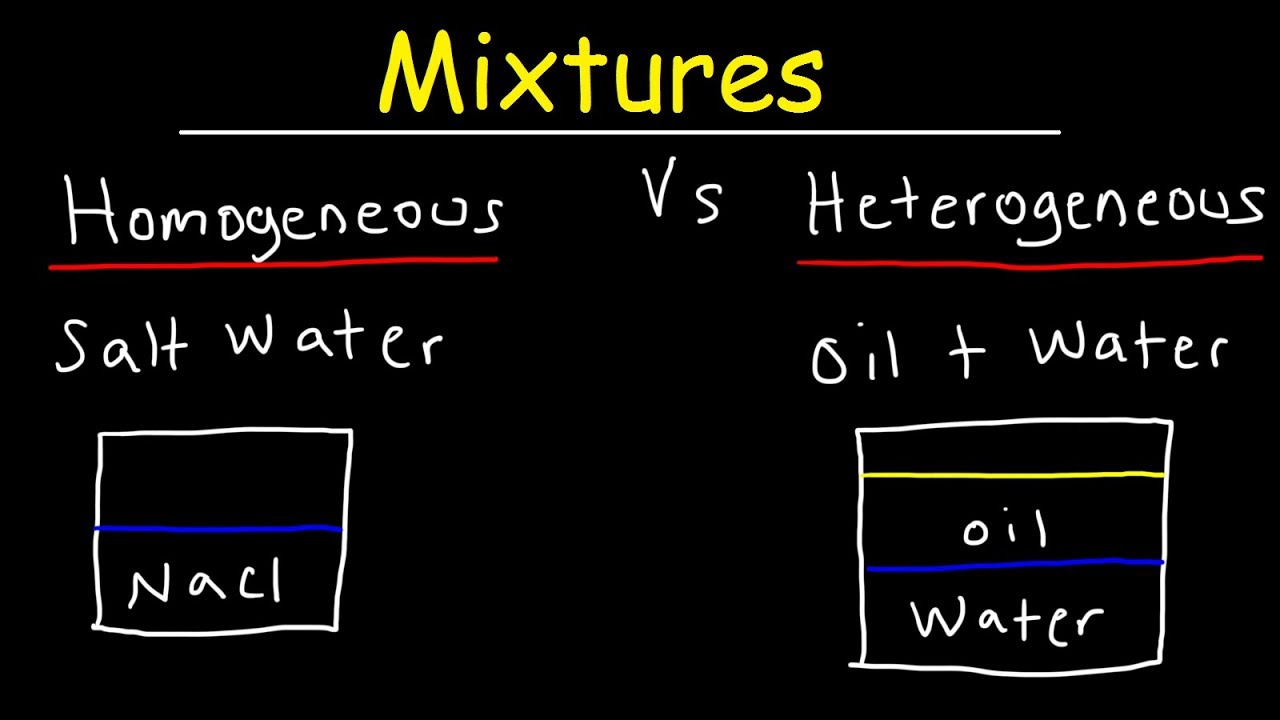



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube




Chapter Two Properties Of Matter Matter Pure Substance Elementcompoundmixture Homogeneous Mixture Solution Heterogeneous Mixture Colloidsuspension Classification Ppt Download
Eg wine, beer, gelatin, water and alcohol Heterogeneous Mixtures Unlike homogeneous mixtures, in these it is very easy to identify, even to the naked eye, which are the different components that make them up This makes it much easier to separate these mixtures Eg water and oil, water and sand Mixtures can be characterized by being separable by mechanical means eg heat, filtration, gravitational sorting, centrifugation etc Mixtures can be either homogeneous or heterogeneous' a mixture in which constituents are distributed uniformly is called homogeneous, such as salt in water, otherwise it is called heterogeneous, such as sand in water When two or more substances (compounds or elements) mix together without showing any chemical change, they are called mixtures It generally has two major types based on the mixing of substances, such as homogeneous mixtures and heterogeneous mixtures You can say that in mixtures, there is a thorough mechanical blending without any
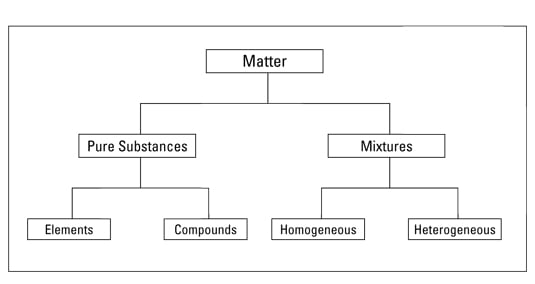



How To Distinguish Pure Substances And Mixtures Dummies




Elements Mixtures And Compounds Lab Studocu
Heterogeneous Mixtures are those in which participants or components can be distinguished easily their composition is nonuniform also having their phase integrated irregularly and unevenly therefore it is possible to distinguish their phases with relative ease Heterogeneous Mixture Heterogeneous mixtures are do not have a uniform distribution of components The composition of components does vary from sample to sample Sometimes, the components are visible by naked eyes and can be handpicked, for example, the mixture of sand and gravel or the mixture of marble balls and stonesWood is composed of a number of compounds such as lignin, cellulose, water, hemicellulose, etc The relative composition of wood varies from plant to plant Unlike a compound, wood does not have a fixed chemical formula



May 13 Notes




Elements Compounds And Mixtures Activity Pdf Mixture Chemical Substances
Start studying Element, Compound, Homogeneous or Heterogeneous Mixture Learn vocabulary, terms, and more with flashcards, games, and other study tools Mixtures in two or more phases are heterogeneous mixtures Examples include ice cubes in a drink, sand and water, and salt and oil Is paper heterogeneous or homogeneous?Students gain a better understanding of the different types of materials as pure substances and mixtures and learn to distinguish between homogeneous and heterogeneous mixtures by discussing an assortment of example materials




1 Which Of The Below Figures Represents A Gaseous Chegg Com




What Are The Types Of Pure Substances And Mixtures A Plus Topper
There are three wellknown phases gases, liquids, and solids (A less wellknown phase is plasma which is a gas that is so hot that it has enough energy for some electrons to leave their atoms;Helium is an element It is made of off helium atoms in somebody were given seawater Seawater is a heterogeneous mixture It is heterogeneous mixture because apart from sand and water, which might have a uniformed composition tour, they send another impurities in seawater, which make it a heterogeneous mixture Homogeneous mixtures and heterogeneous mixtures Element a element is the simplest pure substance Air and its constituents Molecules of elements or compounds Displaying top 8 worksheets found for elements and compounds Distribute a periodic table of elements to each student discuss the combinations of elements and the compounds and mixtures




Elements Compounds And Mixtures Diagram Quizlet
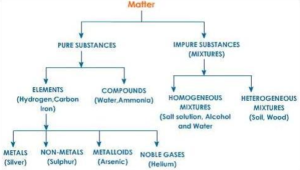



Heterogeneous Mixture Archives The Fact Factor
An example of the latter would be a mixture of water, octane, and silicone greaseIf they are substances, subclassify them as compounds or elements6 rows A heterogeneous mixture is a type of mixture that allows the components to be seen as two or



Elements Compounds And Mixtures Course Hero
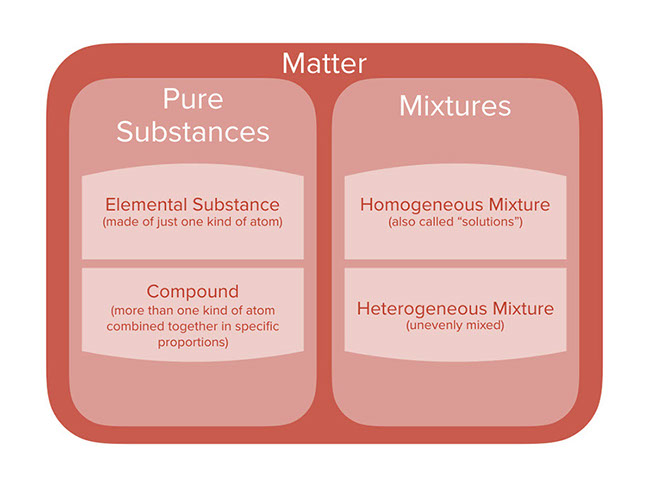



Lesson Categories Of Chemicals And Mixtures
The rainy atmosphere is a mixture of the air, which is a gas, with liquid rain droplets There are patches of dense liquid water falling down through the less dense air It's an obviously heterogeneous mixture because there are two states, or phases of matter liquid and gas Importantly, on a rainy day, visibility is limited




Elements Compounds And Mixtures Classification Of Matter Student Note Organizer
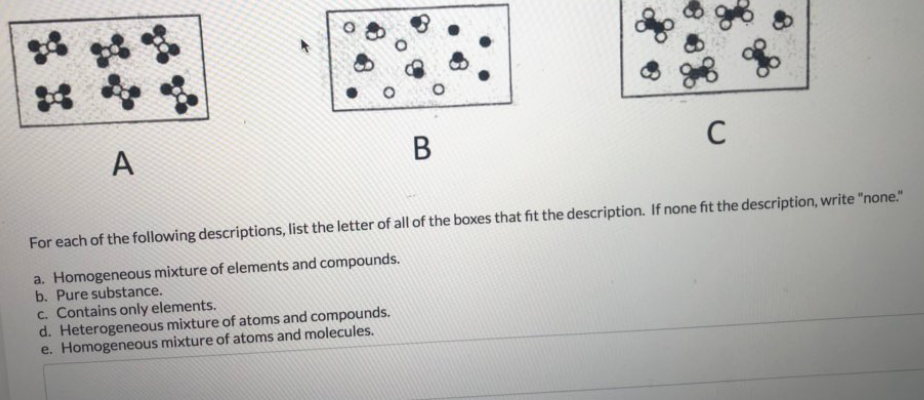



For Each Of The Following Descriptions List The Chegg Com




Question 19 Each Circle In The Diagrams Represents Chegg Com



Elements Compounds And Mixtures Sas




Examples Of Homogeneous Mixtures Solid Liquid And Gas
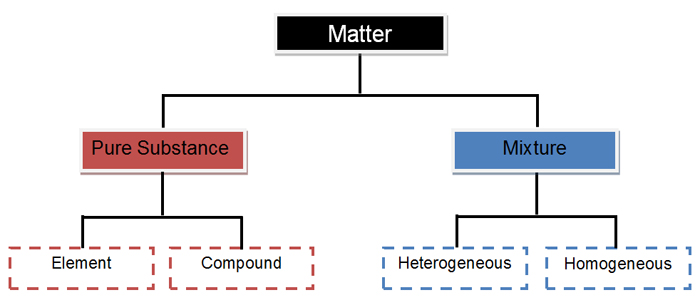



Matter And Energy Elements Versus Compounds Texas Gateway
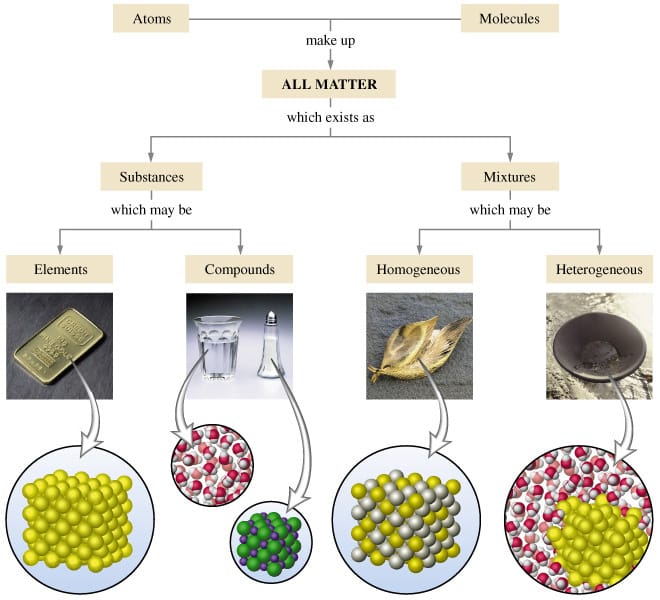



Classifying Matter Schoolworkhelper



Mixtures 8th Grade Science Brogden




Phases And Classification Of Matter Chemistry




Element Compound And Mixture Powerpoint Slides




Pure Substances And Mixtures Classification Of Matter Youtube



Www Dimanregional Org Site Handlers Filedownload Ashx Moduleinstanceid 39 Dataid 2719 Filename Cycle 1 chem 1 lesson 5 Pdf




Elements Compounds And Mixtures Word Search Wordmint



1




3 4 Classifying Matter According To Its Composition Chemistry Libretexts




Elements Compounds And Mixtures 3 Kinds Of Matter




Pure Substances And Mixtures Elements Compounds And Mixtures Pure Products Compounds And Mixtures
(120).jpg)



Chemistry Mixtures Elements Compounds Quiz Proprofs Quiz



Elements Compounds And Mixtures Presentation Chemistry




Elements Compounds And Mixtures Science Quiz Quizizz




Classify The Following As Pure Substances Or Mixtures Separate The Pure Substances Into Elements Compounds And Divide The Mixtures Into Homogeneous And Heterogeneous I Air Ii Milk Iii Graphite Iv Gasoline




2 2 Mixtures Classification Of Matter Siyavula



Sci8u1l2




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com




Elements Componds And Mixtures By Emily Carlevato Infographic



Matter Elements Compounds Mixtures Matter Matter Is Anything




Matter And Elements Final



1




2 1 Matter



Pure Substances And Mixtures Neds Declassified




If Each Geometric Shape Represents A Different Type Of Atom And Atoms That Are Touching Have Brainly Com
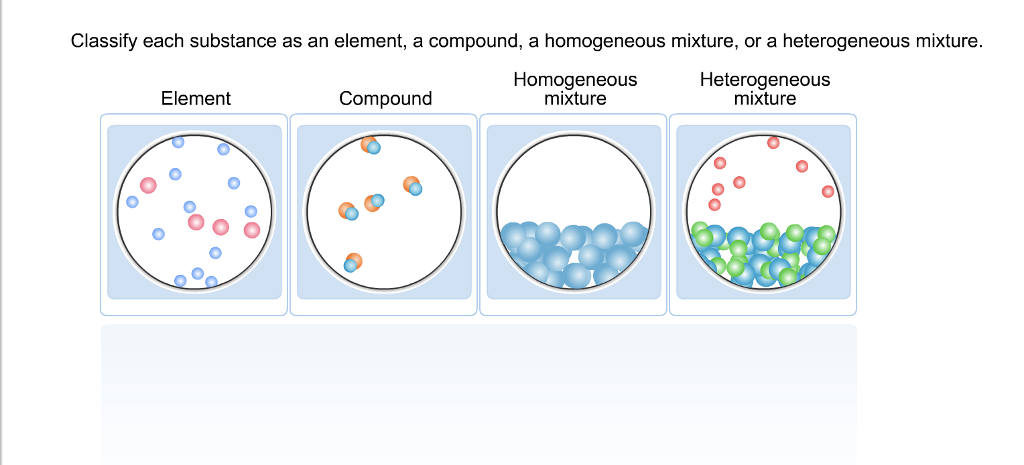



Classify Each Substance As An Element A Compound A Chegg Com



Heterogeneous Mixture Ppt Video Online Download



Solved 1 A Classify Each Of The Following As An Element A Compound A Homogeneous Mixture Or A Heterogeneous Mixture



Classification Of Matter



Http Www Whsd Net Userfiles 1729 Classes B1 making concept maps Pdf



Heterogeneous And Homogeneous Mixture Differences Videos Examples



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Matter 02 Elements Molecules Compounds Mixtures Graphic Organizers



Www Mrsd Org Cms Lib Nh Centricity Domain 245 Element compound mixture notes Pdf



Elements Mixtures And Compounds School Chemistry



1
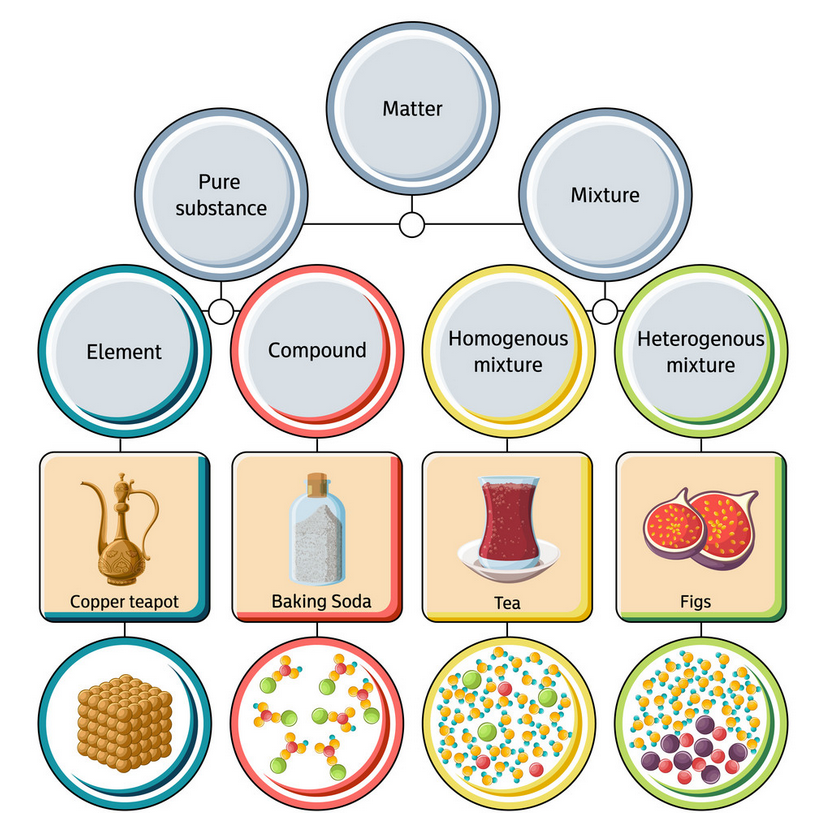



Classifying Matter




Ppt Elements Compounds Homogeneous Mixtures Heterogeneous Mixtures Powerpoint Presentation Id




Chemistry For Kids Chemical Mixtures




Elements Compounds Mixtures Composition Of Matter Pure Substances
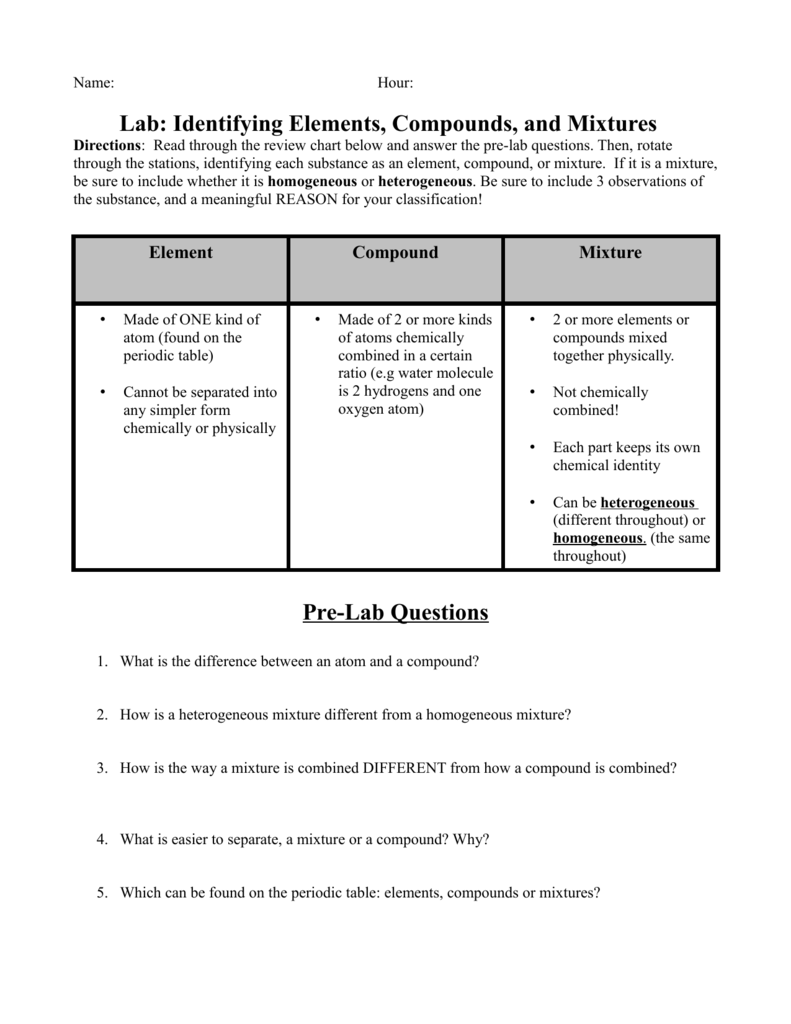



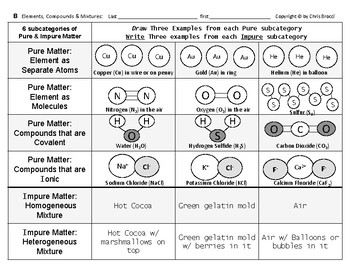
Lab Identifying Elements Compounds And Mixtures Pre



Elements Compounds And Mixtures Interactive Worksheet By Tanisha Easterling Wizer Me




Elements Compounds Mixtures Science Quiz Quizizz
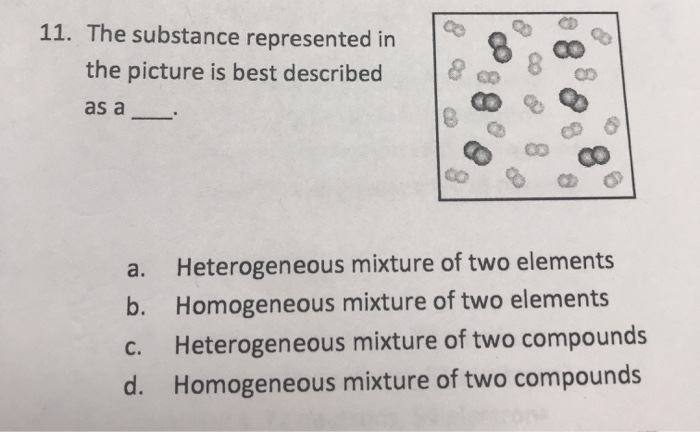



11 The Substance Represented In The Picture Is Best Chegg Com
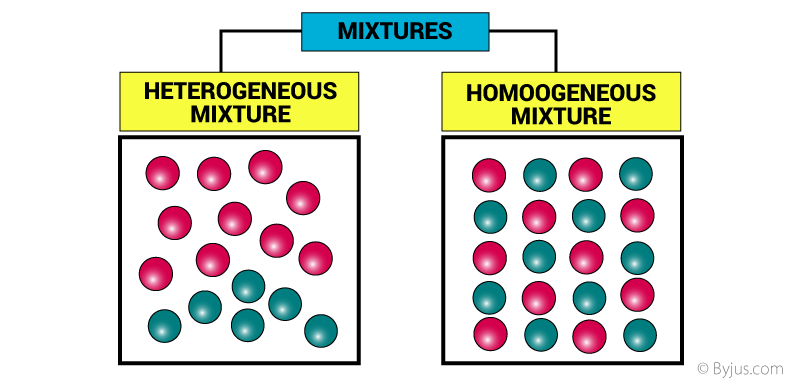



What Is A Mixture Definition Properties Examples Types With Videos




Pure Substances Elements Compounds Homogenous Heterogenous Mixture Examples And Problems Youtube



Http Sites Isdschools Org Grade6 Remote Learning Resources Useruploads 05 11 Science6 Schimmelsmartwynn May13 Pdf




Elements And Compounds Chemistry 8
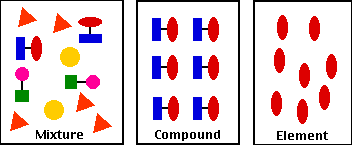



Mixtures And Compounds




Elements Compounds And Mixtures Worksheet Elements Compounds And Mixtures Compounds And Mixtures Compounds




2 3 Pure Substances Classification Of Matter Siyavula



1




Matter Mixtures Compounds And Elements



Lecture For Wednesday August 19th
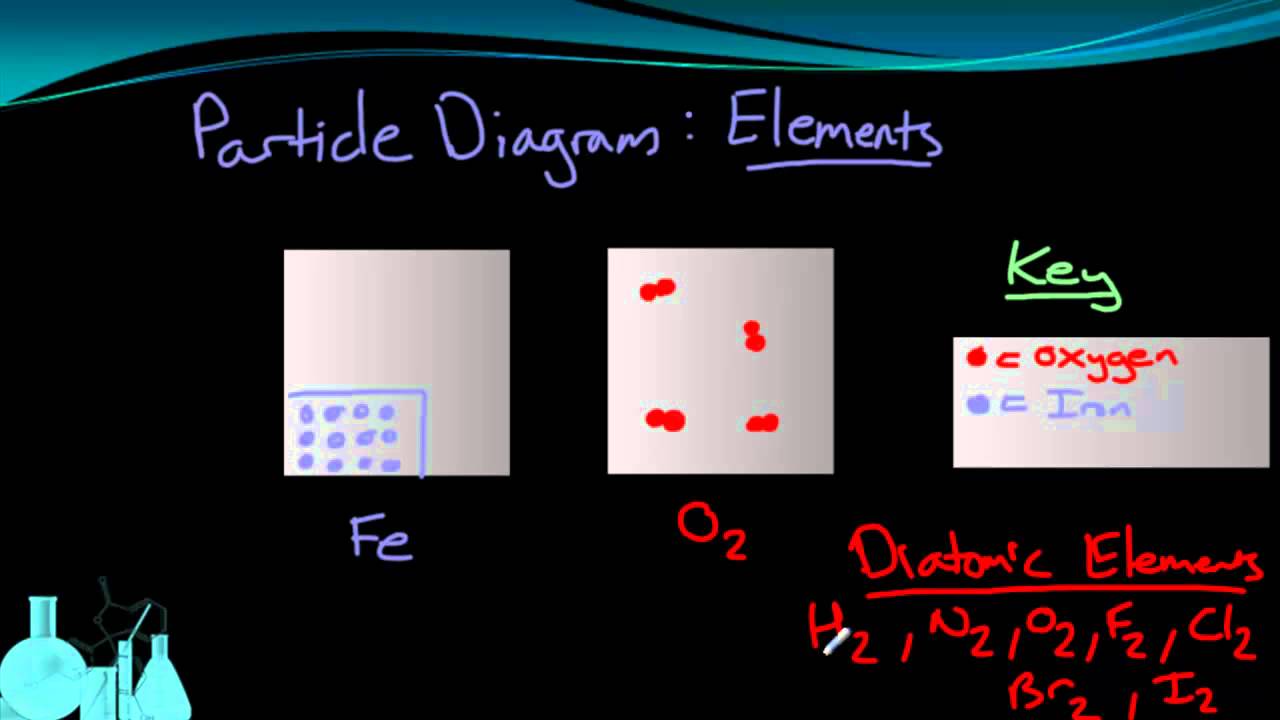



Classification Of Matter Elements Compounds Mixtures Introductory Chemistry




What Are The Types Of Pure Substances Compounds Elements Videos
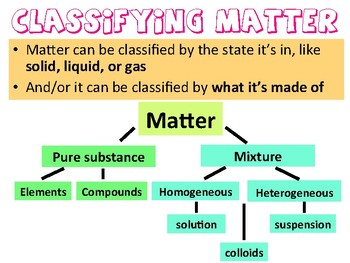



Homogeneous Heterogeneous Mixtures Powerpoint By Doxie Designs




Classification Of Matter Chemistrygod
.png?revision=1)



1 3 Classification Of Matter Chemistry Libretexts




Mixtures And Compounds




What Is A Heterogeneous Mixture Definition And Examples




10 Heterogeneous And Homogeneous Mixtures




Mixtures Compounds And Elements Siyavula Textbooks Grade 10 Physical Science Openstax Cnx



Www Unf Edu Michael Lufaso Chem45 Chapter1 Pdf




Chapter 1 Section 2




Element Compound And Mixture Powerpoint Slides




Compound Vs Mixture Difference And Comparison Diffen



Explain I Pure Substances Vs Mixtures Uths Demo Course




Mixtures A Mixture Is A Form Of Matter That Is Composed Of Two Or More Elements Two Or More Compounds Or Of Elements And Compounds Ppt Download
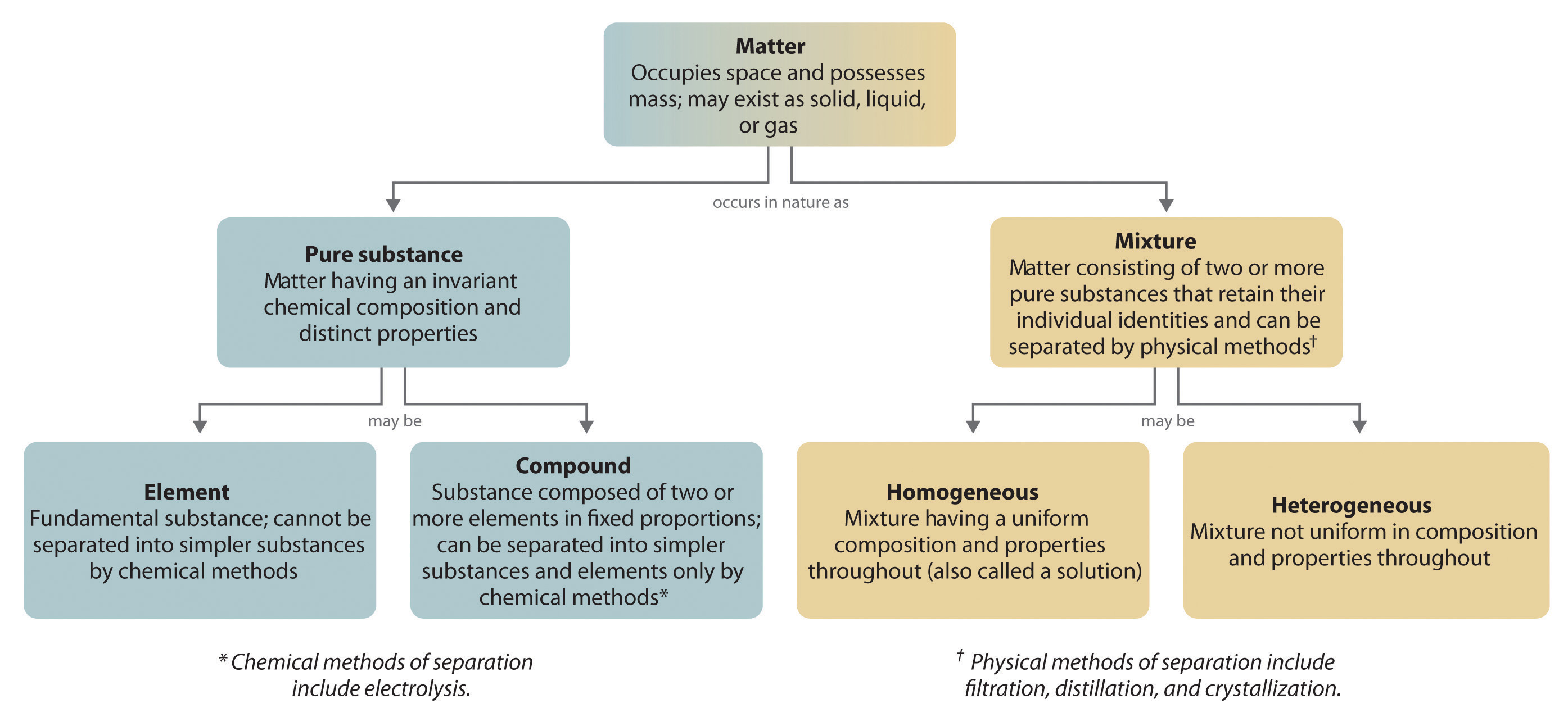



1 3 Classification Of Matter Chemistry Libretexts




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
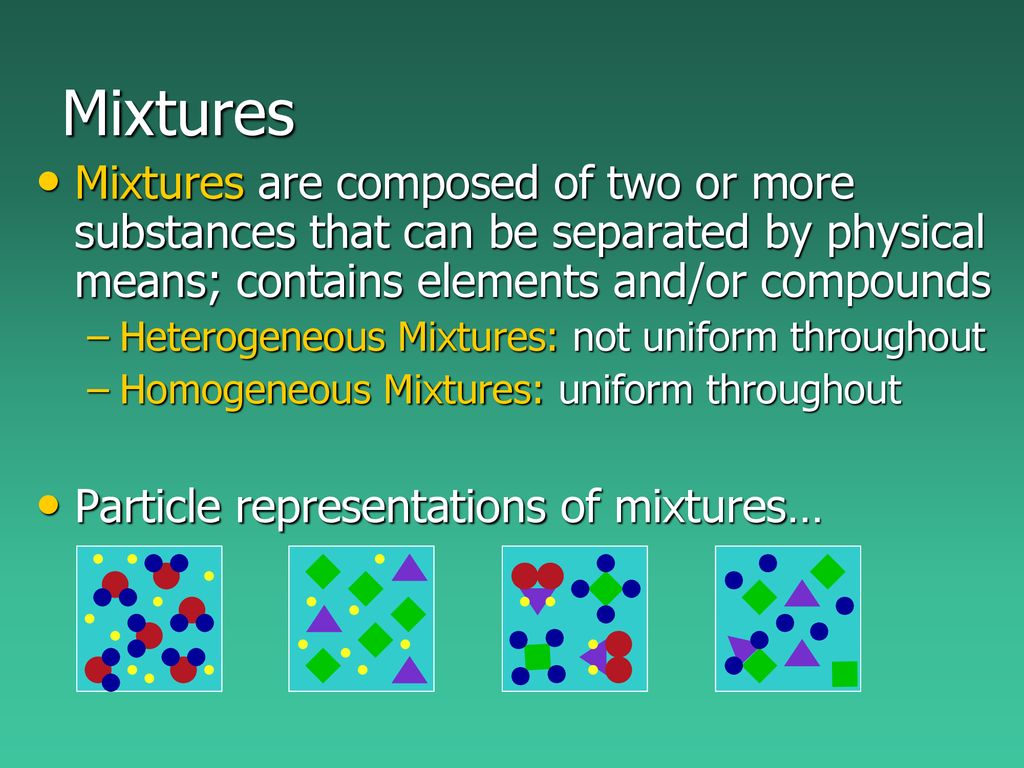



Unit 5 Pure Substances Mixtures Ppt Download
コメント
コメントを投稿